Diffusion and selectivity of water confined within metal–organic nanotubes.†
Abstract
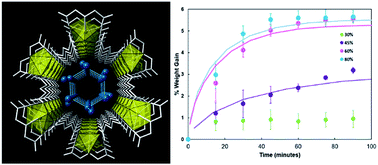
Behavior of nanoconfined water in porous materials has important implications for the development of advanced water purification and storage. In the current study, the kinetics of water sorption from the vapor phase into a metal organic nanotube ((C4N2H6)[(UO2)(C4O4NH5)(C4O4NH6)]·2H2O (UMON)) are investigated with varying relative humidity. The UMON compound contains nanoconfined water molecules arranged in an ice-like array along the length of its one-dimensional pore and exhibits complete specificity to liquid water. Total hydration of the material is observed upon exposure to relative humidity of 60% or higher. Water uptake curves are modeled as diffusion and irreversible condensation in the pore, which leads to a modeled diffusion coefficient of (1.2 ± 0.6) × 10−12 cm2 s−1 for water in UMON nanochannels. This value is much lower than observed for other porous material and is most similar to water diffusivity in low-density amorphous ice. In addition, on exposure to various solvent vapors, the UMON material maintained specificity for water in the gas phase.


 Please wait while we load your content...
Please wait while we load your content...