Melatonin-directed micellization: a case for tryptophan metabolites and their classical bioisosteres as templates for the self-assembly of bipyridinium-based supramolecular amphiphiles in water†
Abstract
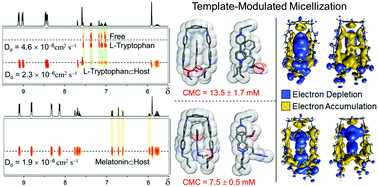
The bulk solution properties of amphiphilic formulations are derivative of their self-assembly into higher ordered supramolecular assemblies known as micelles and of their ordering at the air–water interface. Exerting control over the surface-active properties of amphiphiles and their propensity to aggregate in pure water is most often fine-tuned by covalent modification of their molecular structure. Nevertheless structural constraints which limit the performance of amphiphiles do emerge when trying to develop more sophisticated systems which undergo for example, shape-defined controlled assembly and/or respond to external stimuli. In this regard, the template-modulated assembly of the so-called “supramolecular amphiphiles” continues to make progress ordering molecules that otherwise have very little to no driving force to aggregate in a prescribed manner in aqueous solutions. Herein we describe the template-modulated micellization and ordering at the air–water interface of bipyridinium-based supramolecular amphiphiles triggered by host–guest interactions with high specificity for the neurotransmitter melatonin over its biosynthetic synthon L-tryptophan and the thermodynamic parameters governing the template-modulated micellization process. When bound to the bipyridinium units of micellized surfactant molecules, melatonin effectively serves as “molecular glue” capable of lowering the CMC by 52% as compared to untemplated solutions. Analysis of this system suggests that a hallmark of donor–acceptor template-modulated micellization in water is a strong positively correlated temperature dependence of the CMC and the absence of a U-shaped CMC–temperature curve. Our findings make a case for the incorporation of L-tryptophan-based metabolites and their classical synthetic pharmaceutical bioisosteres as potential targets/components of donor–acceptor CT-based supramolecular amphiphile systems/materials operating in water.


 Please wait while we load your content...
Please wait while we load your content...