Spontaneous symmetry breaking of charge-regulated surfaces†
Abstract
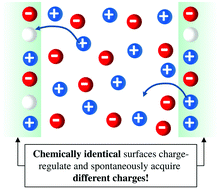
The interaction between two chemically identical charge-regulated surfaces is studied using the classical density functional theory. In contrast to common expectations and assumptions, under certain realistic conditions we find a spontaneous emergence of disparate charge densities on the two surfaces. The surface charge densities can differ not only in their magnitude, but quite unexpectedly, even in their sign, implying that the electrostatic interaction between the two chemically identical surfaces can be attractive instead of repulsive. Moreover, an initial symmetry with equal charge densities on both surfaces can also be broken spontaneously upon decreasing the separation between the two surfaces. The origin of this phenomenon is a competition between the adsorption of ions from the solution to the surface and the interaction between the adsorbed ions already on the surface. These findings are fundamental for the understanding of the forces between colloidal objects and, in particular, they are bound to strongly influence the present picture of protein interaction.


 Please wait while we load your content...
Please wait while we load your content...