Aqueous solution behaviour and solubilisation properties of octadecyl cationic gemini surfactants and their comparison with their amide gemini analogues†
Abstract
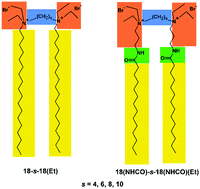
Gemini surfactants 18–s–18(Et), comprised of two ethylammonium headgroups and two alkyl tails with m = 18 carbon atoms with spacers of s = 4, 6, 8 and 10 linking the headgroups (alkanediyl-α,ω-bis(diethyloctadecylammonium bromides)), were obtained. Their aqueous solution behaviour, including adsorption at the interface and aggregation in solution, was followed by tensiometric, conductometric and spectroscopic methods. The critical micelle concentration (CMC) of the surfactants decreased with increasing spacer length. The size of 18–s–18(Et) aggregates formed at concentrations of 10 and 40 CMC measured by DLS varied with the elongation of the spacer. Visualisation of aggregated surfactant structures at 40 CMC by cryo-TEM evidenced the formation of different morphologies depending on spacer length. Gemini with s = 4 formed elongated, cylindrical micelles, while geminis of s = 6, 8 and 10 self-assembled into vesicles. The ability of the studied geminis to solubilise hydrophobic dye Sudan I in water was determined as a function of surfactant concentration, demonstrating their high efficiency. Results for 18–s–18(Et) geminis were compared with those previously obtained for their analogues containing an amide group placed between headgroups and tails. The significant impact of amide groups on the surface activity and aggregation properties of gemini surfactants was evidenced and is related to hydrogen-bond formation by amide-containing compounds.


 Please wait while we load your content...
Please wait while we load your content...