Effect of HU protein on the conformation and compaction of DNA in a nanochannel
Abstract
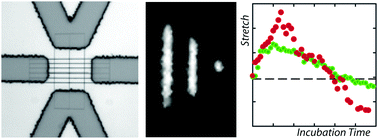
The effect of the heat unstable nucleoid structuring protein HU on the conformation of single DNA molecules confined in a nanochannel was investigated with fluorescence microscopy. Pre-incubated DNA molecules contract in the longitudinal direction of the channel with increasing concentration of HU. This contraction is mainly due to HU-mediated bridging of distal DNA segments and is controlled by channel diameter as well as ionic composition and strength of the buffer. For over-threshold concentrations of HU, the DNA molecules compact into an condensed form. Divalent magnesium ions facilitate, but are not required for bridging nor condensation. The conformational response following exposure to HU was investigated with a nanofluidic device that allows an in situ change in environmental solution conditions. The stretch of the nucleoprotein complex first increases, reaches an apex in ∼20 min, and subsequently decreases to an equilibrium value pertaining to pre-incubated DNA molecules after ∼2 h. This observation is rationalised in terms of a time-dependent bending rigidity by structural rearrangement of bound HU protein followed by compaction through bridging interaction. Results are discussed in regard to previous results obtained for nucleoid associated proteins H-NS and Hfq, with important implications for protein binding related gene regulation.


 Please wait while we load your content...
Please wait while we load your content...