A pH and salt dually responsive emulsion in the presence of amphiphilic macromolecules†
Abstract
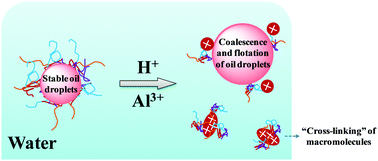
A pH and salt dually responsive emulsion has been designed on the basis of a novel amphiphilic macromolecule. It was found that the water separation of an oil-in-water emulsion reached up to ∼60% after standing for 10 min at low pH. 2-(Diethylamino)ethyl methacrylate (DEA) residues were found to induce the macromolecules to protonate and to be hydrophilic at pH values between 2 and 6, resulting in dewetting from oil droplet surfaces in water. Besides, the macromolecules form aggregates with different structures at the water/oil interface, depending on the pH value or salt concentration of the emulsion system, enabling the system to be demulsified in response to the pH or salt stimulus. The experimental results also showed that with the addition of aluminium chloride at 100 mg L−1, the water separation was about 70% after 20 min. A possible mechanism with respect to demulsifying was proposed on the basis of an “ion bridge” among sodium acrylate (SA) residues, inducing the macromolecules to “cross-link” and become insoluble, and leading to oil/water separation. Furthermore, at a fixed pH of 5, addition of salt to the aqueous dispersion increased the degree of oil–water interfacial activity and batch emulsions were significantly unstable to coalesce at a low salinity of 25–50 mg L−1. This finding presents a new manipulation on emulsion stability and potential applications in the fields of oil recovery, wastewater treatment, sludge removal, and so on.


 Please wait while we load your content...
Please wait while we load your content...