Proton coupled electron transfer from Co3O4 nanoparticles to photogenerated Ru(bpy)33+: base catalysis and buffer effect†
Abstract
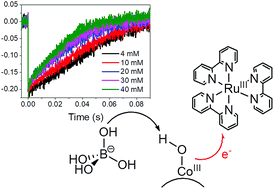
Co3O4 nanoparticles in the spinel crystalline structure are among the most promising catalysts for the water oxidation reaction, displaying remarkable activity under electrochemical and light-assisted conditions. In the presence of Ru(bpy)32+ as a photosensitizer (bpy = 2,2′-bipyridine) and Na2S2O8 as an electron acceptor, 5 ± 1 nm size Co3O4 nanoparticles show a slow primary electron transfer (ET) to photogenerated Ru(III), occurring in a timescale of tens of milliseconds. We demonstrate herein that (i) photo-oxidation of Co3O4 NPs by Ru(III) involves transformation of surface Co(III)–OH sites to formal Co(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, along a proton-coupled electron-transfer (PCET) pathway; (ii) the rate of the process depends on pH, and on the nature and concentration of the buffer; (iii) borate promotes general base catalysis of the PCET; and (iv) inhibition of the PCET is observed at high buffer concentration, due to H3BO3 poisoning of the surface Co sites, resulting in depletion of the O2 evolution activity.
O, along a proton-coupled electron-transfer (PCET) pathway; (ii) the rate of the process depends on pH, and on the nature and concentration of the buffer; (iii) borate promotes general base catalysis of the PCET; and (iv) inhibition of the PCET is observed at high buffer concentration, due to H3BO3 poisoning of the surface Co sites, resulting in depletion of the O2 evolution activity.


 Please wait while we load your content...
Please wait while we load your content...