Water-tolerant lithium metal cycling in high lithium concentration phosphonium-based ionic liquid electrolytes†
Abstract
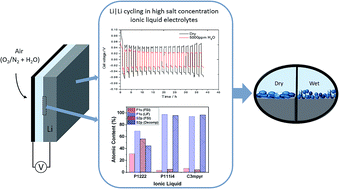
Cycling stability at high capacities and water-tolerance are two key properties for the operation of high-capacity lithium (Li) metal–air batteries. Here, we have demonstrated the cycling of Li metal at high rates and high capacities in a newly developed family of ionic liquids based on quaternary alkylphosphonium cations and the bis(fluorosulfonyl)imide anion. A high LiFSI salt concentration of 50 mol% gave the most favourable combination of performance and water-tolerance when compared to 33 mol% and 20 mol%. These high salt content electrolytes exhibited stable cycling at 1 mA cm−2, in 1 h steps for up to 250 cycles at room temperature in the presence of up to 5000 ppm water. The two smallest cations, triethylmethylphosphonium (P1222) and trimethylisobutylphosphonium (P111i4), showed significantly superior cycling capabilities than the larger tributylmethylphosphonium (P1444) and trihexyltetradecylphosphonium (P66614) cations. Furthermore, the two small phosphonium cations supported high current densities and more stable long-term cycling than the N-methyl-N-propylpyrrolidinium cation (C3mpyr), which is of similar molecular weight. Fourier transform infra-red spectroscopy was used to characterise the composition of electrode surface layers, while ex situ X-ray photoelectron spectroscopy showed that the presence of water affects the amount of decomposition products at the electrode surface. These benchmark cycling results represent a significant step forward in the development of water-tolerant electrolytes for Li metal-based batteries.


 Please wait while we load your content...
Please wait while we load your content...