Two-electron oxidation of water to form hydrogen peroxide catalysed by silicon-porphyrins†
Abstract
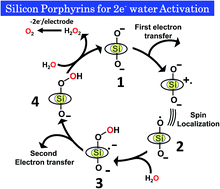
The second most earth abundant atom incorporated meso-tetrapyridylporphyrinatesilicon(IV) (SiTPyP) as an electrochemical catalyst was newly synthesized. The two axially ligated water molecules on SiTPyP and the four meso-substituted pyridyl nitrogens suffer eight-step protonations and deprotonations. Our detailed pKa study indicated that the two axially ligated water molecules were fully deprotonated to exist as O−–Si–O− under the condition of pH > 2. Under basic conditions, SiTPyP(O−)2 exerted the two-electron oxidation of water to form hydrogen peroxide as the primary product in aqueous acetonitrile, which was initiated through a one-electron oxidation process on the electrode with faradaic yield of 84–92%.
- This article is part of the themed collections: 3rd International Solar Fuels Conference and International Conference on Artificial Photosynthesis and Artificial Photosynthesis - From Sunlight to Fuels and Valuable Products for a Sustainable Future


 Please wait while we load your content...
Please wait while we load your content...