Electrochemical reduction of carbon dioxide with a molecular polypyridyl nickel complex†
Abstract
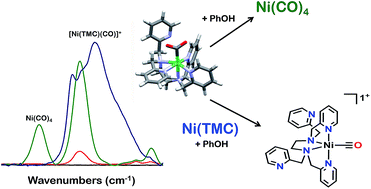
The synthesis and reactivity of a molecular nickel(II) complex 1 with the polypyridyl ligand framework N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) under electrochemically reducing conditions in the presence of CO2 is reported. Cyclic voltammetry (CV), infrared spectroelectrochemistry (IR-SEC) and electrolysis experiments suggest this Ni complex is competent at mediating the two-electron reduction of CO2 to CO and H2O with phenol as an added proton donor, but is subsequently prone to rapid degradation as Ni(CO)4. This deleterious pathway was shown to be mitigated by the inclusion of the CO scavenger [Ni(TMC)]2+, although this requires stoichiometric inclusion for each catalyst turnover and does not significantly improve the observed catalytic current densities.


 Please wait while we load your content...
Please wait while we load your content...