Preparation of mixed-ion and inorganic perovskite films using water and isopropanol as solvents for solar cell applications†
Abstract
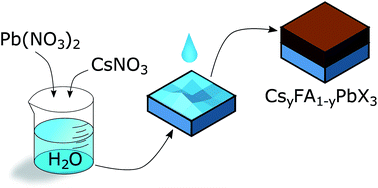
Presently, the most efficient lead halide perovskite solar cells are manufactured by using high-boiling point organic solvents to dissolve the perovskite precursor materials prior to the perovskite formation. Previously, efforts have been made to exchange the said solvents for water with some success. Herein, we build on that work to develop a procedure for synthesising perovskite absorbers using only water and isopropanol as solvents. Our technique can be utilised for fabricating many different perovskite compositions, organic and inorganic. The technique is based on the high solubility of metal nitrates, such as lead(II) nitrate and caesium(I) nitrate, in water and, respectively, their poor solubilities in isopropanol. The inclusion of CsNO3 to Pb(NO3)2 films does not result in a phase separation of the perovskite material as one would expect when using lead(II) halide precursor films. Using the perovskite composition Cs0.1FA0.9Pb(I0.83Br0.17)3 we were able to reach an average solar cell power conversion efficiency of 13.0%. Furthermore, the technique can be applied to many different perovskite compositions making it appealing for large-scale manufacturing of perovskite solar cells.


 Please wait while we load your content...
Please wait while we load your content...