Influence of additives in a PVDF-based solid polymer electrolyte on conductivity and Li-ion battery performance
Abstract
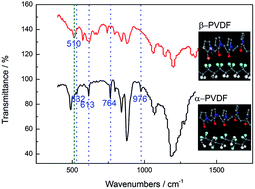
Polyvinylidene fluoride (PVDF) was used as a membrane matrix, and then many groups of experiments with different additives were carried out to investigate the factor that affects conductivity of the solid polymer electrolyte (SPE). Impedance measurements were performed using an electrochemical work station. Data obtained through alternating current (AC) impedance measurements revealed that the conductivity of the blending membrane was improved. The presence of hydroxyl end-groups and some electron-donating groups in the additives was helpful for increasing the conductivity. In particular, when PVP and EDTA were added, the conductivity of the polymer electrolyte could achieve a value of 7.19 × 10−4 S cm−1 at 25 °C and a lithium ion transference number of 0.49. The electrochemical stability window was about 4.3 V (vs. Li+/Li).


 Please wait while we load your content...
Please wait while we load your content...