Theoretical studies on the reaction kinetics of methyl crotonate with hydroxyl radical†
Abstract
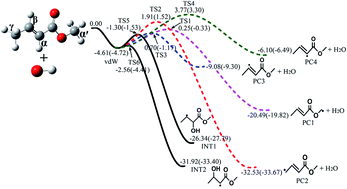
The potential energy surfaces (PES) for the reactions of methyl crotonate (MC) with hydroxyl radical, including H-abstraction and OH-addition, were explored by the QCISD(T)/CBS//M062x/6-311++G(d,p) and CBS-QB3 methods, respectively. The subsequent isomerization and β-scission reactions of the produced primary radicals were also investigated using these methods. The phenomenological rate coefficients of these reactions were predicted over 500 to 1800 K and 0.001 to 100 atm via solving the time-dependent master equations based on Rice–Ramsperger–Kassel–Marcus theory. The rate coefficients of the two dominant H-abstraction channels at 1 atm were expressed as the sum of two modified Arrhenius equations (units: cm3 per molecule per s and cal mol−1): k1 (MC + OH → PC1 + H2O) = 2.29 × 10−4T−2.02 exp(−10 204/RT) + 7.31 × 10−13T0.1 exp(−819/RT), k2 (MC + OH → PC2 + H2O) = 1.83 × 10−2T−2.39 exp(−10 360/RT) + 1.27 × 10−9T−0.57 exp(−2858/RT). The sub-mechanisms of MC + OH and its primary radicals were validated using a previously described combustion model; the updated model well reproduced the previous experimental results. The present rate coefficients provide valuable kinetic data to improve our understanding of the combustion mechanism of MC and can be used as a reliable reference for further investigation of practical fuels.


 Please wait while we load your content...
Please wait while we load your content...