Tetrazine-mediated bioorthogonal prodrug–prodrug activation†
Abstract
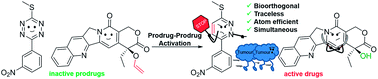
The selective and biocompatible activation of prodrugs within complex biological systems remains a key challenge in medical chemistry and chemical biology. Herein we report, for the first time, a dual prodrug activation strategy that fully satisfies the principle of bioorthogonality by the symbiotic formation of two active drugs. This dual and traceless prodrug activation strategy takes advantage of the INVDA chemistry of tetrazines (here a prodrug), generating a pyridazine-based miR21 inhibitor and the anti-cancer drug camptothecin and offers a new concept in prodrug activation.


 Please wait while we load your content...
Please wait while we load your content...