Enantioselective aza-Friedel–Crafts reaction of furan with α-ketimino esters induced by a conjugated double hydrogen bond network of chiral bis(phosphoric acid) catalysts†
Abstract
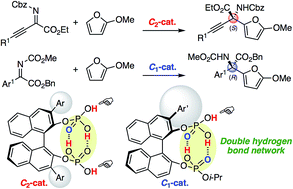
Chiral C2- and C1-symmetric BINOL-derived bis(phosphoric acid) catalysts, which have OP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(OH)2/OP(
O)(OH)2/OP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(OH)(OR) moieties at the 2,2′-positions, were developed and used for the enantioselective aza-Friedel–Crafts reaction of 2-methoxyfuran with α-ketimino esters for the first time. The intramolecular conjugated double hydrogen bond network is a key to increasing the Brønsted acidity and preventing deactivation of the catalysts. Highly functionalized α-amino acid derivatives with a chiral quaternary carbon center could be transformed into versatile optically active N- and O-heterocycles and an α-aryl-substituted serine.
O)(OH)(OR) moieties at the 2,2′-positions, were developed and used for the enantioselective aza-Friedel–Crafts reaction of 2-methoxyfuran with α-ketimino esters for the first time. The intramolecular conjugated double hydrogen bond network is a key to increasing the Brønsted acidity and preventing deactivation of the catalysts. Highly functionalized α-amino acid derivatives with a chiral quaternary carbon center could be transformed into versatile optically active N- and O-heterocycles and an α-aryl-substituted serine.


 Please wait while we load your content...
Please wait while we load your content...