The coordination chemistry of CmIII, AmIII, and AcIII in nitrate solutions: an actinide L3-edge EXAFS study†‡
Abstract
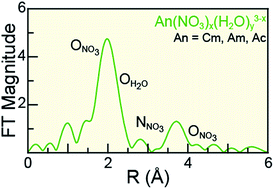
Understanding actinide(III) (AnIII = CmIII, AmIII, AcIII) solution-phase speciation is critical for controlling many actinide processing schemes, ranging from medical applications to reprocessing of spent nuclear fuel. Unfortunately, in comparison to most elements in the periodic table, AnIII speciation is often poorly defined in complexing aqueous solutions and in organic media. This neglect – in large part – is a direct result of the radioactive properties of these elements, which make them difficult to handle and acquire. Herein, we surmounted some of the handling challenges associated with these exotic 5f-elements and characterized CmIII, AmIII, and AcIII using AnIII L3-edge X-ray absorption spectroscopy (XAS) as a function of increasing nitric acid (HNO3) concentration. Our results revealed that actinide aquo ions, An(H2O)x3+ (x = 9.6 ± 0.7, 8.9 ± 0.8, and 10.0 ± 0.9 for CmIII, AmIII, and AcIII), were the dominant species in dilute HNO3 (0.05 M). In concentrated HNO3 (16 M), shell-by-shell fitting of the extended X-ray fine structure (EXAFS) data showed the nitrate complexation increased, such that the average stoichiometries of Cm(NO3)4.1±0.7(H2O)5.7±1.3(1.1±0.2)−, Am(NO3)3.4±0.7(H2O)5.4±0.5(0.4±0.1)−, and Ac(NO3)2.3±1.7(H2O)8.3±5.2(0.7±0.5)+ were observed. Data obtained at the intermediate HNO3 concentration (4 M) were modeled as a linear combination of the 0.05 and 16 M spectra. For all three metals, the intermediate models showed larger contributions from the 0.05 M HNO3 spectra than from the 16 M HNO3 spectra. Additionally, these efforts enabled the Cm–NO3 and Ac–NO3 distances to be measured for the first time. Moreover, the AnIII L3-edge EXAFS results, contribute to the growing body of knowledge associated with CmIII, AmIII, and AcIII coordination chemistry, in particular toward advancing understanding of AnIII solution phase speciation.


 Please wait while we load your content...
Please wait while we load your content...