Formal Giese addition of C(sp3)–H nucleophiles enabled by visible light mediated Ni catalysis of triplet enone diradicals†
Abstract
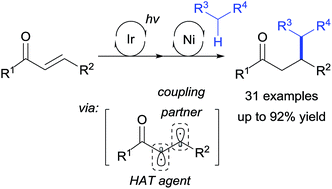
An unprecedented utilization of triplet excited enones in Ni-catalysis enabled a formal Giese addition of C(sp3)–H nucleophiles. This mechanism-based approach has greatly widened the reaction scope, allowing the synthesis of previously inaccessible structures. In this process, the enone diradical acted as two distinct reaction centers, participating in both metalation and hydrogen atom transfer, ultimately furnishing a range of formal Giese addition products in a highly general context. This reaction provides complementary access to traditional 1,4-addition reactions of enones, with a future perspective to develop triplet diradical-based transition metal catalysis.


 Please wait while we load your content...
Please wait while we load your content...