Reagent-dictated site selectivity in intermolecular aliphatic C–H functionalizations using nitrogen-centered radicals†
Abstract
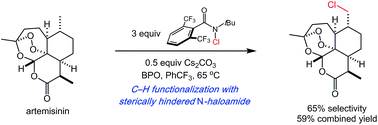
The site selectivities of intermolecular, aliphatic C–H bond functionalizations are central to the value of these transformations. While the scope of these reactions continues to expand, the site selectivities remain largely dictated by the inherent reactivity of the substrate C–H bonds. Herein, we introduce reagent-dictated site selectivity to intermolecular aliphatic C–H functionalizations using nitrogen-centered amidyl radicals. Simple modifications of the amide lead to high levels of site selectivity in intermolecular C–H functionalizations across a range of simple and complex substrates. DFT calculations demonstrate that the steric demand of the reacting nitrogen-centered radical is heavily affected by the substitution pattern of the starting amide. Optimization of transition state structures consistently indicated higher reagent-dictated steric selectivities using more hindered amides, consistent with experimental results.


 Please wait while we load your content...
Please wait while we load your content...