Microwave-assisted functionalization of the Aurivillius phase Bi2SrTa2O9: diol grafting and amine insertion vs. alcohol grafting†
Abstract
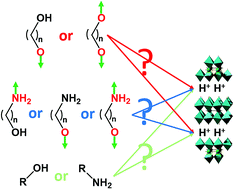
Microwave-assisted functionalization of the layered Aurivillius phase Bi2SrTa2O9 by alcohols is thoroughly investigated. The grafting of linear aliphatic and bulky alcohols is studied as a function of the starting material, underlining the importance of the prefunctionalization of the layered perovskite, for instance by butylamine. In addition, the functionalization by α,ω-alkanediols is explored. α,ω-alkanediols bearing long alkyl chains (nC > 3) adopt an unprecedented pillaring arrangement, whereas 1,3-propanediol and ethyleneglycol adopt a bilayer arrangement, only one out of the two hydroxyl groups being coordinated. Finally, the reactivities of alcohols and amines towards insertion are compared: the preferential reactivity of the two functional groups appears to be strongly dependent of the reaction conditions, and especially of the water content. This study is further extended to the case of amino-alcohol insertion. In this case, the amine group is preferentially bound, but it is possible to control the grafting of the alcohol moiety, thus going from a bilayer arrangement to a pillaring one. This work is of particular importance to be able to functionalize easily and rapidly layered oxides with elaborated molecules, bearing several different potentially reactive groups.


 Please wait while we load your content...
Please wait while we load your content...