Photocatalytic reverse polarity Povarov reaction†
Abstract
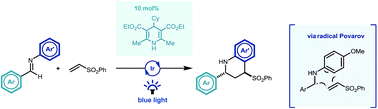
A visible light mediated iridium photocatalysed reverse polarity Povarov reaction of aryl imines and electron deficient alkenes is described. Operating via a putative nucleophilic α-amino radical, generated by a proton coupled electron transfer process, addition to a range of conjugated electron deficient alkene substrates affords substituted tetrahydroquinoline products in high yields and with typically good to excellent diastereoselectivity in favor of the trans diastereoisomer. Sub-stoichiometric quantities of Hantzsch ester were found to be key to initiate the overall redox-neutral, free radical cyclization cascade. This new reaction complements existing two electron Lewis acid mediated variants and expands the capabilities of imine umpolung chemistry to synthetically relevant cyclisation methodology.


 Please wait while we load your content...
Please wait while we load your content...