Site-selective C–C modification of proteins at neutral pH using organocatalyst-mediated cross aldol ligations†
Abstract
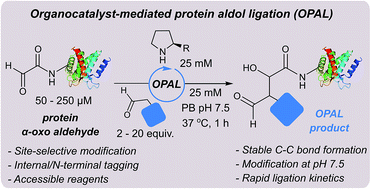
The bioconjugation of proteins with small molecules has proved an invaluable strategy for probing and perturbing biological mechanisms. The general use of chemical methods for protein functionalisation can be limited however by the requirement for complicated reaction partners to be present in large excess, and harsh conditions which are incompatible with many protein scaffolds. Herein we describe a site-selective organocatalyst-mediated protein aldol ligation (OPAL) that affords stable carbon–carbon linked bioconjugates at neutral pH. OPAL enables rapid modification of proteins using simple aldehyde probes in minimal excess, and is utilised here in the affinity tagging of proteins in cell lysate. Furthermore we demonstrate that the β-hydroxy aldehyde OPAL product can be functionalised again at neutral pH in a tandem organocatalyst-mediated oxime ligation. This tandem strategy is showcased in the ‘chemical mimicry’ of a previously inaccessible natural dual post-translationally modified protein integral to the pathogenesis of the neglected tropical disease Leishmaniasis.
- This article is part of the themed collections: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis, The ChemRxiv Collection and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...