Preparative microdroplet synthesis of carboxylic acids from aerobic oxidation of aldehydes†
Abstract
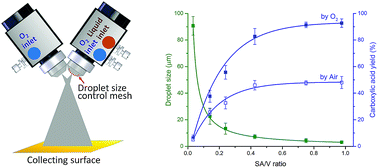
Single liquid-phase and liquid–liquid phase reactions in microdroplets have shown much faster kinetics than that in the bulk phase. This work extends the scope of microdroplet reactions to gas–liquid reactions and achieves preparative synthesis. We report highly efficient aerobic oxidation of aldehydes to carboxylic acids in microdroplets. Molecular oxygen plays two roles: (1) as the sheath gas to shear the aldehyde solution into microdroplets, and (2) as the sole oxidant. The dramatic increase of the surface-area-to-volume ratio of microdroplets compared to bulk solution, and the efficient mixing of gas and liquid phases using spray nozzles allow effective mass transfer between aldehydes and molecular oxygen. The addition of catalytic nickel(II) acetate is shown to accelerate further microdroplet reactions of this kind. We show that aliphatic, aromatic, and heterocyclic aldehydes can be oxidized to the corresponding carboxylic acids in a mixture of water and ethanol using the nickel(II) acetate catalyst, in moderate to excellent yields (62–91%). The microdroplet synthesis is scaled up to make it preparative. For example, aerobic oxidation of 4-tert-butylbenzaldehyde to 4-tert-butylbenzoic acid was achieved at a rate of 10.5 mg min−1 with an isolated product yield of 66%.


 Please wait while we load your content...
Please wait while we load your content...