Direct heterobenzylic fluorination, difluorination and trifluoromethylthiolation with dibenzenesulfonamide derivatives†
Abstract
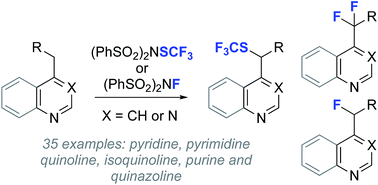
Functionalization of heterocyclic scaffolds with mono- or difluoroalkyl groups provides unique opportunities to modulate drug pKa, influence potency and membrane permeability, and attenuate metabolism. While advances in the addition of fluoroalkyl radicals to heterocycles have been made, direct C(sp3)–H heterobenzylic fluorination is comparatively unexplored. Here we demonstrate both mono- and difluorination of a range of alkyl heterocycles using a convenient process that relies on transient sulfonylation by the electrophilic fluorinating agent N-fluorobenzenesulfonimide. We also report heterobenzylic trifluoromethylthiolation and 18F-fluorination, providing a suite of reactions for late-stage C(sp3)–H functionalization of drug leads and radiotracer discovery.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...