Transition-metal-free decarboxylative bromination of aromatic carboxylic acids†
Abstract
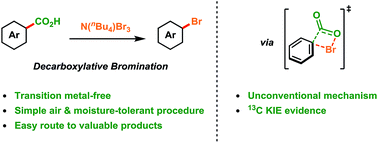
Methods for the conversion of aliphatic acids to alkyl halides have progressed significantly over the past century, however, the analogous decarboxylative bromination of aromatic acids has remained a longstanding challenge. The development of efficient methods for the synthesis of aryl bromides is of great importance as they are versatile reagents in synthesis and are present in many functional molecules. Herein we report a transition metal-free decarboxylative bromination of aromatic acids. The reaction is applicable to many electron-rich aromatic and heteroaromatic acids which have previously proved poor substrates for Hunsdiecker-type reactions. In addition, our preliminary mechanistic study suggests that radical intermediates are not involved in this reaction, which is in contrast to classical Hunsdiecker-type reactivity. Overall, the process demonstrates a useful method for producing valuable reagents from inexpensive and abundant starting materials.
- This article is part of the themed collections: Most popular 2018-2019 organic chemistry articles and 2019 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...