Diastereoisomers of l-proline-linked trityl-nitroxide biradicals: synthesis and effect of chiral configurations on exchange interactions†
Abstract
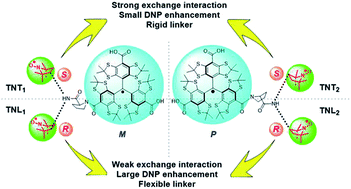
The exchange (J) interaction of organic biradicals is a crucial factor controlling their physiochemical properties and potential applications and can be modulated by changing the nature of the linker. In the present work, we for the first time demonstrate the effect of chiral configurations of radical parts on the J values of trityl-nitroxide (TN) biradicals. Four diastereoisomers (TNT1, TNT2, TNL1 and TNL2) of TN biradicals were synthesized and purified by the conjugation of a racemic (R/S) nitroxide with the racemic (M/P) trityl radical viaL-proline. The absolute configurations of these diastereoisomers were assigned by comparing experimental and calculated electronic circular dichroism (ECD) spectra as (M, S, S) for TNT1, (P, S, S) for TNT2, (M, S, R) for TNL1 and (P, S, R) for TNL2. Electron paramagnetic resonance (EPR) results showed that the configuration of the nitroxide part instead of the trityl part is dominant in controlling the exchange interactions and the order of the J values at room temperature is TNT1 (252 G) > TNT2 (127 G) ≫ TNL2 (33 G) > TNL1 (14 G). Moreover, the J values of TNL1/TNL2 with the S configuration in the nitroxide part vary with temperature and the polarity of solvents due to their flexible linker, whereas the J values of TNT1/TNT2 are almost insensitive to these two factors due to the rigidity of their linkers. The distinct exchange interactions between TNT1,2 and TNL1,2 in the frozen state led to strongly different high-field dynamic nuclear polarization (DNP) enhancements with ε = 7 for TNT1,2 and 40 for TNL1,2 under 800 MHz DNP conditions.


 Please wait while we load your content...
Please wait while we load your content...