A porous, electrically conductive hexa-zirconium(iv) metal–organic framework†
Abstract
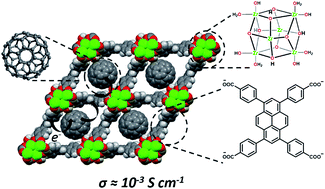
Engendering electrical conductivity in high-porosity metal–organic frameworks (MOFs) promises to unlock the full potential of MOFs for electrical energy storage, electrocatalysis, or integration of MOFs with conventional electronic materials. Here we report that a porous zirconium-node-containing MOF, NU-901, can be rendered electronically conductive by physically encapsulating C60, an excellent electron acceptor, within a fraction (ca. 60%) of the diamond-shaped cavities of the MOF. The cavities are defined by node-connected tetra-phenyl-carboxylated pyrene linkers, i.e. species that are excellent electron donors. The bulk electrical conductivity of the MOF is shown to increase from immeasurably low to 10−3 S cm−1, following fullerene incorporation. The observed conductivity originates from electron donor–acceptor interactions, i.e. charge-transfer interactions – a conclusion that is supported by density functional theory calculations and by the observation of a charge-transfer-derived band in the electronic absorption spectrum of the hybrid material. Notably, the conductive version of the MOF retains substantial nanoscale porosity and continues to display a sizable internal surface area, suggesting potential future applications that capitalize on the ability of the material to sorb molecular species.
- This article is part of the themed collections: Most popular 2018-2019 materials chemistry articles and 2019 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...