Protein surface functionalisation as a general strategy for facilitating biomimetic mineralisation of ZIF-8†
Abstract
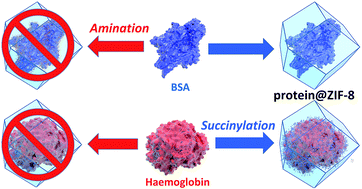
The durability of enzymes in harsh conditions can be enhanced by encapsulation within metal–organic frameworks (MOFs) via a process called biomimetic mineralisation. Herein we show that the surface charge and chemistry of a protein determines its ability to seed MOF growth. We demonstrate that chemical modification of amino acids on the protein surface is an effective method for systematically controlling biomimetic mineralisation by zeolitic imidazolate framework-8 (ZIF-8). Reaction of surface lysine residues with succinic (or acetic) anhydride facilitates biomimetic mineralisation by increasing the surface negative charge, whereas reaction of surface carboxylate moieties with ethylenediamine affords a more positively charged protein and hinders the process. Moreover, computational studies confirm that the surface electrostatic potential of a protein is a good indicator of its ability to induce biomimetic mineralisation. This study highlights the important role played by protein surface chemistry in encapsulation and outlines a general method for facilitating the biomimetic mineralisation of proteins.
- This article is part of the themed collections: Most popular 2018-2019 materials chemistry articles, 2018 ChemSci Pick of the Week Collection, 2018 Chemical Science HOT Article Collection and Collection to celebrate our diverse and global authorship


 Please wait while we load your content...
Please wait while we load your content...