A flexible, redox-active macrocycle enables the electrocatalytic reduction of nitrate to ammonia by a cobalt complex†
Abstract
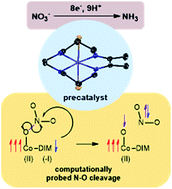
The cobalt macrocycle complex [Co(DIM)Br2]+ (DIM = 2,3-dimethyl-1,4,8,11-tetraazacyclotetradeca-1,3-diene) is an electrocatalyst for the selective reduction of nitrate to ammonia in aqueous solution. The catalyst operates over a wide pH range and with very high faradaic efficiency, albeit with large overpotential. Experimental investigations, supported by electronic structure calculations, reveal that catalysis commences when nitrate binds to the two-electron reduced species CoII(DIM−), where cobalt and the macrocycle are each reduced by a single electron. Several mechanisms for the initial reduction of nitrate to nitrite were explored computationally and found to be feasible at room temperature. The reduced DIM ligand plays an important role in these mechanisms by directly transferring a single electron to the bound nitrate substrate, activating it for further reactions. These studies further reveal that the DIM macrocycle is critical to nitrate reduction, specifically its combination of redox non-innocence, hydrogen-bonding functionality and flexibility in coordination mode.


 Please wait while we load your content...
Please wait while we load your content...