Aldehyde group driven aggregation-induced enhanced emission in naphthalimides and its application for ultradetection of hydrazine on multiple platforms†
Abstract
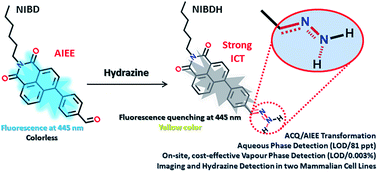
Restriction of intramolecular motion (RIM) of rigid aromatic cores is the most universal mechanism so far that can successfully explain almost all AIE/AIEEgenic systems. By designing two novel naphthalimide derivatives (NIB and NIBD), we experimentally demonstrated the effect of a single formyl group that can efficiently transform an aggregation caused quenching (ACQ) system (NIB) into an AIEEgenic system (NIBD) by strengthening the RIM process. Besides, the newly designed naphthalimide AIEEgen (NIBD) accomplished ultrasensitive detection of hydrazine at the parts per trillion level (LOD/81 ppt) in aqueous media with high selectivity and enormous improvement over the existing state of the art. An exceptional sensitivity is also achieved in the vapor phase (LOD/0.003%) using a Whatman paper strip based portable device for simple and cost-effective on-site detection. The detection mechanism involved a reaction-based spontaneous formation of a non-fluorescent hydrazone Schiff base derivative (NIBDH). The in vitro potentiality of the AIEEgenic probe was also demonstrated in two mammalian cell lines i.e. HeLa (human cervical cancer cell line) and HEK293T (Human embryonic kidney cell line that expresses a mutant version of the SV40 large T antigen). Owing to the highly selective formation of the hydrazone Schiff base complex with hydrazine, NIBD responds to the existence of hydrazine in both these cell lines without any interference from other biologically rich metal ions and amino acids. These outcomes could initiate a much wider use of formyl group induced condensed state emission and a key hypothesis that could generate newer avenues for ACQ to AIEE transformations for several practical applications including hydrazone Schiff base complexation for probing and manipulating hydrazine biology associated with several metabolic activities.
- This article is part of the themed collections: Celebrating the Chemical Science in India - Leaders in the Field Symposium, Most popular 2018-2019 materials chemistry articles and 2018 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...