Total synthesis of the reported structure of ceanothine D via a novel macrocyclization strategy†
Abstract
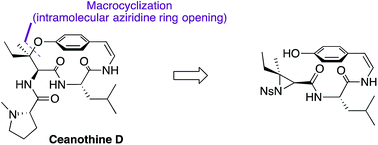
The first total synthesis of the reported structure of ceanothine D, a cyclopeptide alkaloid found in red root, was achieved using a highly convergent synthetic strategy. Highlights of the synthesis include the first concomitant macrocyclization and formation of the unique chiral tertiary alkyl-aryl ether bond with complete regio- and stereo-control in the presence of a sensitive Z-enamide moiety to access the strained para-cyclophane present in its structure. This synthetic strategy may be broadly applicable in the generation of other structurally similar cyclopeptide alkaloids, enabling further biological and chemical investigations.
- This article is part of the themed collections: New reactivity in organic chemistry and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...