Direct catalytic enantioselective amination of ketones for the formation of tri- and tetrasubstituted stereocenters†
Abstract
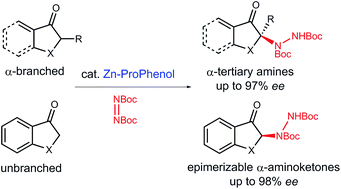
Herein, we report a Zn–ProPhenol catalyzed direct asymmetric amination reaction of unactivated aryl and vinyl ketones using di-tert-butyl azodicarboxylate as a cheap and practical electrophilic nitrogen source. Importantly, this methodology works with both α-branched and unbranched ketones for the construction of tri- and tetrasubstituted N-containing stereocenters. The reaction can be run at gram-scale with low catalyst loadings and features a recoverable and reusable ligand. Finally, the enantioenriched hydrazine products can be readily converted into versatile building blocks such as α-amino carbonyl compounds and β-amino alcohols.


 Please wait while we load your content...
Please wait while we load your content...