Near infrared two-photon-excited and -emissive dyes based on a strapped excited-state intramolecular proton-transfer (ESIPT) scaffold†
Abstract
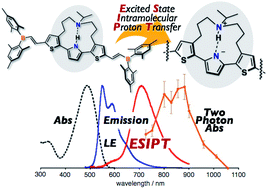
Fluorophores that can undergo excited-state intramolecular proton transfer (ESIPT) represent promising scaffolds for the design of compounds that show red-shifted fluorescence. Herein, we disclose new near infrared-emissive materials based on a dialkylamine-strapped 2,5-dithienylpyrrole as an ESIPT scaffold. The introduction of electron-accepting units to the terminal positions of this scaffold generates acceptor–π–donor–π–acceptor (A–π–D–π–A) type π-conjugated compounds. Following the ESIPT, the electron-donating ability of the core scaffold increases, which results in a substantially red-shifted emission in the NIR region, while increasing the oscillator strength. The electron-accepting units play a vital role to achieve intense and red-shifted emission from the ESIPT state. The strapped dialkylamine chain that forms an intramolecular hydrogen bond is also essential to induce the ESIPT. Moreover, an extended A–π–D–π–A skeleton enables two-photon excitation with the NIR light. One of the derivatives that satisfy these features, i.e., borylethenyl-substituted 5, exhibited an intense NIR emission in polar solvents such as acetone (λem = 708 nm, ΦF = 0.55) with a strong two-photon-absorption band in the NIR region.


 Please wait while we load your content...
Please wait while we load your content...