Engaging sulfinate salts via Ni/photoredox dual catalysis enables facile Csp2–SO2R coupling†
Abstract
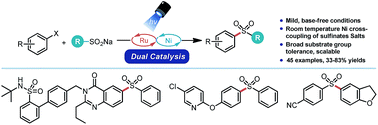
This report details the development and implementation of a strategy to construct aryl- and heteroaryl sulfones via Ni/photoredox dual catalysis. Using aryl sulfinate salts, the C–S bond can be forged at room temperature under base-free conditions. An array of aryl- and heteroaryl halides are compatible with this approach. The broad tolerance and mild nature of the described reaction could potentially be employed to prepare sulfones with biological relevance (e.g., in bioconjugation, drug substance synthesis, etc.) as demonstrated in the synthesis of drug-like compounds or their precursors. When paired with existing Ni/photoredox chemistry for Csp3–Csp2 cross-coupling, an array of diverse sulfone scaffolds can be readily assembled from bifunctional electrophiles. A mechanistic manifold consistent with experimental and computational data is presented.
- This article is part of the themed collection: Most popular 2018-2019 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...