Step-growth titanium-catalysed dehydropolymerisation of amine–boranes†
Abstract
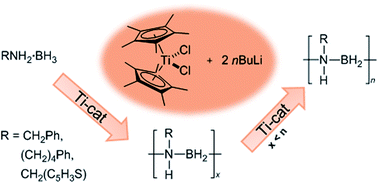
Precatalysts active for the dehydropolymerisation of primary amine–boranes are generally based on mid or late transition metal. We have found that the activity of the precatalyst system formed from CpR2TiCl2 and 2nBuLi towards the dehydrogenation of the secondary amine–borane Me2NH·BH3, to yield the cyclic diborazane [Me2N–BH2]2, increases dramatically with increasing electron-donating character of the cyclopentadienyl rings (CpR). Application of the most active precatalyst system (CpR = η-C5Me5) to the primary amine–borane MeNH2·BH3 enabled the first synthesis of high molar mass poly(N-methylaminoborane), [MeNH–BH2]n, the BN analogue of polypropylene, by an early transition metal such as catalyst. Significantly, unlike other dehydropolymerization precatalysts for MeNH2·BH3 such as [Ir(POCOP)H2], skeletal nickel, and [Rh(COD)Cl]2, the Ti precatalyst system was also active towards a range of substrates including BzNH2·BH3 (Bz = benzyl) yielding high molar mass polymer. Moreover, in contrast to the late transition metal catalysed dehydropolymerisation of MeNH2·BH3 and also the Ziegler–Natta polymerisation of olefins, studies indicate that the Ti-catalyzed dehydropolymerization reactions proceed by a step-growth rather than a chain-growth mechanism.


 Please wait while we load your content...
Please wait while we load your content...