Similar ligand–metal bonding for transition metals and actinides? 5f1 U(C7H7)2−versus 3dn metallocenes†
Abstract
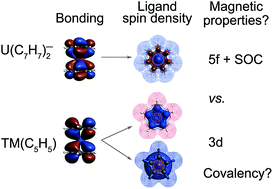
U(C7H7)2− is a fascinating 5f1 complex whose metal–ligand bonding was assigned in the literature as being very similar to 3d7 cobaltocene, based on a crystal-field theoretical interpretation of the experimental magnetic resonance data. The present work provides an in-depth theoretical study of the electronic structure, bonding, and magnetic properties of the 5f1 U(C7H7)2−vs. 3d metallocenes with V, Co, and Ni, performed with relativistic wavefunction and density functional methods. The ligand to metal donation bonding in U(C7H7)2− is strong and in fact similar to that in vanadocene, in the sense that the highest occupied arene orbitals donate electron density into empty metal orbitals of the same symmetry with respect to the rotational axis (3dπ for V, 5fδ for U), but selectively with α spin (↑). For Co and Ni, the dative bonding from the ligands is β spin (↓) selective into partially filled 3dπ orbitals. In all systems, this spin delocalization triggers spin polarization in the arene σ bonding framework, causing proton spin densities opposite to those of the carbons. As a consequence, the proton spin densities and hyperfine coupling constants 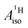 are negative for the Co and Ni complex, but positive for vanadocene. The
are negative for the Co and Ni complex, but positive for vanadocene. The 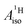 of U(C7H7)2− is negative and similar to that of cobaltocene, but only because of the strong spin–orbit coupling in the actinocene, which causes
of U(C7H7)2− is negative and similar to that of cobaltocene, but only because of the strong spin–orbit coupling in the actinocene, which causes 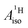 to be opposite to the sign of the proton spin density. The study contributes to a better understanding of actinide 5f vs. transition metal 3d covalency, and highlights potential pitfalls when interpreting experimental magnetic resonance data in terms of covalent bonding for actinide complexes.
to be opposite to the sign of the proton spin density. The study contributes to a better understanding of actinide 5f vs. transition metal 3d covalency, and highlights potential pitfalls when interpreting experimental magnetic resonance data in terms of covalent bonding for actinide complexes.


 Please wait while we load your content...
Please wait while we load your content...