Unexpected solvation-stabilisation of ions in a protic ionic liquid: insights disclosed by a bond energetic study†
Abstract
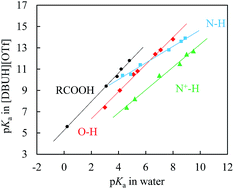
Equilibrium acidities (pKas) of 42 organic acids were precisely determined in protic ionic liquid (PIL) [DBUH][OTf]. Surprisingly, the often seen homoassociation complication during the pKa measurement of O–H acids in DMSO was not detected in [DBUH][OTf], implying that the incipient oxanion should be better solvation-stabilized by the PIL, although its “apparent” dielectric constant is much lower than that of the conventional molecular solvent DMSO. Evidence showing that the solute ions in the PIL are also free from other specific ion associations like ion-pairing is further demonstrated by the identical pKas of protic amine salts bearing largely different counter-anions. Correlations between the RO–H, N–H, N+–H and RCOO–H bond acidities in [DBUH][OTf] and in water revealed different slopes and intercepts for each individual series, suggesting far superior properties of the DBUH+-based PIL for differentiating the solvation effect of various species in structural analysis to the well applied EAN that is known for leveling out differential solvation.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...