Nickel-catalyzed enantioselective allylic alkylation of lactones and lactams with unactivated allylic alcohols†
Abstract
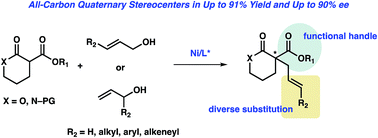
The first nickel-catalyzed enantioselective allylic alkylation of lactone and lactam substrates to deliver products bearing an all-carbon quaternary stereocenter is reported. The reaction, which utilizes a commercially available chiral bisphosphine ligand, proceeds in good yield with a high level of enantioselectivity (up to 90% ee) on a range of unactivated allylic alcohols for both lactone and lactam nucleophiles. The utility of this method is further highlighted via a number of synthetically useful product transformations.


 Please wait while we load your content...
Please wait while we load your content...