The impact of O-glycan chemistry on the stability of intrinsically disordered proteins†
Abstract
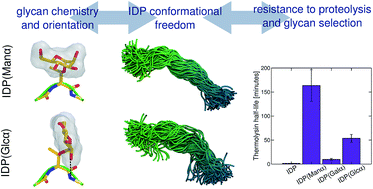
Protein glycosylation is a diverse post-translational modification that serves myriad biological functions. O-linked glycans in particular vary widely in extent and chemistry in eukaryotes, with secreted proteins from fungi and yeast commonly exhibiting O-mannosylation in intrinsically disordered regions of proteins, likely for proteolysis protection, among other functions. However, it is not well understood why mannose is often the preferred glycan, and more generally, if the neighboring protein sequence and glycan have coevolved to protect against proteolysis in glycosylated intrinsically disordered proteins (IDPs). Here, we synthesized variants of a model IDP, specifically a natively O-mannosylated linker from a fungal enzyme, with α-O-linked mannose, glucose, and galactose moieties, along with a non-glycosylated linker. Upon exposure to thermolysin, O-mannosylation, by far, provides the highest extent of proteolysis protection. To explain this observation, extensive molecular dynamics simulations were conducted, revealing that the axial configuration of the C2-hydroxyl group (2-OH) of α-mannose adjacent to the glycan–peptide bond strongly influences the conformational features of the linker. Specifically, α-mannose restricts the torsions of the IDP main chain more than other glycans whose equatorial 2-OH groups exhibit interactions that favor perpendicular glycan–protein backbone orientation. We suggest that IDP stiffening due to O-mannosylation impairs protease action, with contributions from protein–glycan interactions, protein flexibility, and protein stability. Our results further imply that resistance to proteolysis is an important driving force for evolutionary selection of α-mannose in eukaryotic IDPs, and more broadly, that glycan motifs for proteolysis protection likely coevolve with the protein sequence to which they attach.


 Please wait while we load your content...
Please wait while we load your content...