Intramolecular hydrogen-bonding in a cobalt aqua complex and electrochemical water oxidation activity†
Abstract
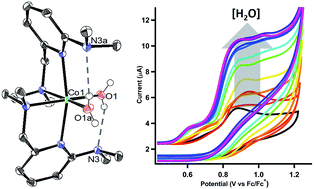
Water oxidation is catalysed in Nature by a redox cofactor embedded in a hydrogen-bonded network designed to orchestrate proton transfer throughout the challenging 4 electron reaction. In order to mimic aspects of this microenvironment, [CoLDMA(CH3CN)2][BF4]2 (2) was synthesized, where LDMA is a dipyridyldiamine ligand with two dimethylamine bases in the secondary coordination sphere. Structural characterization of the corresponding aqua complexes establish hydrogen bonding between the bound water and pendant base(s). Cyclic voltammetry of [CoLDMA(CH3CN)2][BF4]2 (2) reveals enhanced oxidative current upon titration with water and controlled potential electrolysis confirms evolution of O2. The related complex [CoLH(CH3CN)2][BF4]2 (1), which has the same primary coordination environment as 2 but lacks pendant bases, is inactive. The structural and electrochemical studies illustrate the role positioned proton relays can play in promoting redox reactivity.


 Please wait while we load your content...
Please wait while we load your content...