Oxidative β-C–H sulfonylation of cyclic amines†
Abstract
A transition metal-free strategy for the dehydrogenative β-sulfonylation of tertiary cyclic amines is described. N-Iodosuccinimide facilitates regioselective oxidative sulfonylation at C–H bonds positioned β to the nitrogen atom of tertiary amines, installing enaminyl sulfone functionality in cyclic systems. Mild reaction conditions, broad functional group tolerance and a wide substrate scope are demonstrated. The nucleophilic character of the enaminyl sulfone is harnessed, demonstrating potential application for scaffold diversification.
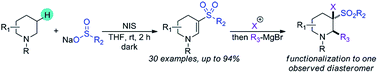


 Please wait while we load your content...
Please wait while we load your content...