Glucose-nucleobase pairs within DNA: impact of hydrophobicity, alternative linking unit and DNA polymerase nucleotide insertion studies†
Abstract
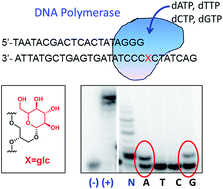
Recently, we studied glucose-nucleobase pairs, a binding motif found in aminoglycoside–RNA recognition. DNA duplexes with glucose as a nucleobase were able to hybridize and were selective for purines. They were less stable than natural DNA but still fit well on regular B-DNA. These results opened up the possible use of glucose as a non-aromatic DNA base mimic. Here, we have studied the incorporation and thermal stability of glucose with different types of anchoring units and alternative apolar sugar-nucleobase pairs. When we explored butanetriol instead of glycerol as a wider anchoring unit, we did not gain duplex thermal stability. This result confirmed the necessity of a more conformationally restricted linker to increase the overall duplex stability. Permethylated glucose-nucleobase pairs showed similar stability to glucoside-nucleobase pairs but no selectivity for a specific nucleobase, possibly due to the absence of hydrogen bonds between them. The three-dimensional structure of the duplex solved by NMR located both, the hydrophobic permethylated glucose and the nucleobase, inside the DNA helix as in the case of glucose-nucleobase pairs. Quantum chemical calculations on glucose-nucleobase pairs indicate that the attachment of the sugar to the DNA skeleton through the OH1 or OH4 positions yields the highest binding energies. Moreover, glucose was very selective for guanine when attached through OH1 or OH4 to the DNA. Finally, we examined DNA polymerase insertion of nucleotides in front of the saccharide unit. KF− polymerase from E. coli inserted A and G opposite glc and 6dglc with low efficiency but notable selectivity. It is even capable of extending the new pair although its efficiency depended on the DNA sequence. In contrast, Bst 2.0, SIII and BIOTAQ™ DNA polymerases seem to display a loop-out mechanism possibly due to the flexible glycerol linker used instead of deoxyribose.


 Please wait while we load your content...
Please wait while we load your content...