Reactivity of highly Lewis acidic diborane(4) towards pyridine and isocyanide: formation of boraalkene–pyridine complex and ortho-functionalized pyridine derivatives†
Abstract
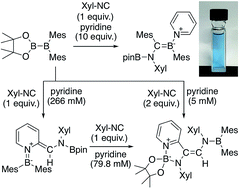
The reaction of pinB-BMes2 (pin = pinacolato, Mes = 2,4,6-Me3C6H2) with Xyl-NC (Xyl = 2,6-Me2C6H3) and pyridine results in the formation of a pyridine-coordinated boraalkene that exhibits an intense color caused by an intramolecular charge-transfer interaction. In the presence of an excess of pyridine, the ortho C–H bond of pyridine was selectively functionalized to afford a quinoid compound or an isocyanide-coupled product. Based on the concentration effect, the reaction stoichiometry, and previously reported DFT calculations, a reaction mechanism that involves several rearrangement reactions was proposed. Using the present method, substituted pyridines and N-heterocycles afforded the corresponding functionalized derivatives. A subsequent hydrolysis of one of the resulting products furnished an aminomethylated pyridine derivative in two steps from parent pyridine.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...