Are multiple oxygen species selective in ethylene epoxidation on silver?†
Abstract
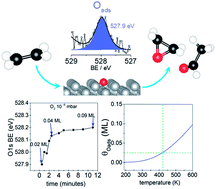
The nature of the oxygen species active in ethylene epoxidation is a long-standing question. While the structure of the oxygen species that participates in total oxidation (nucleophilic oxygen) is known the atomic structure of the selective species (electrophilic oxygen) is still debated. Here, we use both in situ and UHV X-ray Photoelectron Spectroscopy (XPS) to study the interaction of oxygen with a silver surface. We show experimental evidence that the unreconstructed adsorbed atomic oxygen (Oads) often argued to be active in epoxidation has a binding energy (BE) ≤ 528 eV, showing a core-level shift to lower BE with respect to the O-reconstructions, as previously predicted by DFT. Thus, contrary to the frequent assignment, adsorbed atomic oxygen cannot account for the electrophilic oxygen species with an O 1s BE of 530–531 eV, thought to be the active species in ethylene epoxidation. Moreover, we show that Oads is present at very low O-coverages during in situ XPS measurements and that it can be obtained at slightly higher coverages in UHV at low temperature. DFT calculations support that only low coverages of Oads are stable. The highly reactive species is titrated by background gases even at low temperature in UHV conditions. Our findings suggest that at least two different species could participate in the partial oxidation of ethylene on silver.


 Please wait while we load your content...
Please wait while we load your content...