Tuning protein folding in lysosomal storage diseases: the chemistry behind pharmacological chaperones
Abstract
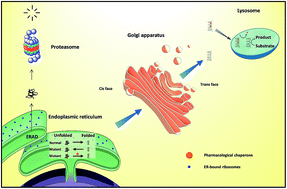
Misfolding of proteins is the basis of several proteinopathies. Chemical and pharmacological chaperones are small molecules capable of inducing the correct conformation of proteins, thus being of interest for human therapeutics. The most recent developments in medicinal chemistry and in the drug development of pharmacological chaperones are discussed, with focus on lysosomal storage diseases.
- This article is part of the themed collections: Most popular 2018-2019 chemical biology articles and Most impactful Chemical Biology articles of 2018


 Please wait while we load your content...
Please wait while we load your content...