High-throughput identification of G protein-coupled receptor modulators through affinity mass spectrometry screening†
Abstract
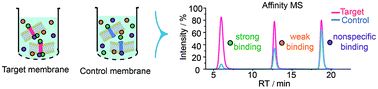
G protein-coupled receptors (GPCRs) represent the largest class of cell surface proteins and thus constitute an important family of therapeutic targets. Therefore, significant effort has been put towards the identification of novel ligands that can modulate the activity of a GPCR target with high efficacy and selectivity. However, due to limitations inherent to the most common techniques for GPCR ligand discovery, there is a pressing need for more efficient and effective ligand screening methods especially for the identification of potential allosteric modulators. Here we present a high-throughput, label-free and unbiased screening approach for the identification of small molecule ligands towards GPCR targets based on affinity mass spectrometry. This new approach features the usage of target-expressing cell membranes rather than purified proteins for ligand screening and allows the detection of both orthosteric and allosteric ligands targeting specific GPCRs. Screening a small compound library with this approach led to the rapid discovery of an antagonist for the 5-HT receptor and four positive allosteric modulators for GLP-1 receptor that were not previously reported.
- This article is part of the themed collections: Celebrating 100 Years of Chemistry at Nankai University and 2018 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...