Tuning the redox non-innocence of a phenalenyl ligand toward efficient nickel-assisted catalytic hydrosilylation†
Abstract
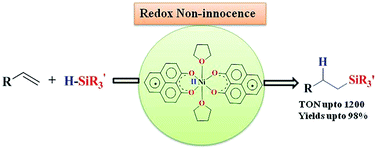
In this report, a ligand-redox assisted catalytic hydrosilylation has been investigated. A phenalenyl ligand coordinated nickel complex has been utilized as an electron reservoir to develop a base metal-assisted catalyst, which very efficiently hydrosilylates a wide variety of olefin substrates under ambient conditions. A mechanistic investigation revealed that a two-electron reduced phenalenyl based biradical nickel complex plays the key role in such catalysis. The electronic structure of the catalytically active biradical species has been interrogated using EPR spectroscopy, magnetic susceptibility measurements, and electronic structure calculations using a DFT method. Inhibition of the reaction by a radical quencher, as well as the mass spectrometric detection of two intermediates along the catalytic loop, suggest that a single electron transfer from the ligand backbone initiates the catalysis. The strategy of utilising the redox reservoir property of the ligand ensures that the nickel is not promoted to an unfavorable oxidation state, and the fine tuning between the ligand and metal redox orbitals elicits smooth catalysis.
- This article is part of the themed collection: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020


 Please wait while we load your content...
Please wait while we load your content...