Nickel-catalyzed coupling reaction of alkyl halides with aryl Grignard reagents in the presence of 1,3-butadiene: mechanistic studies of four-component coupling and competing cross-coupling reactions†
Abstract
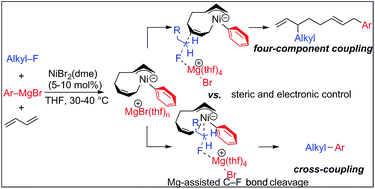
We describe the mechanism, substituent effects, and origins of the selectivity of the nickel-catalyzed four-component coupling reactions of alkyl fluorides, aryl Grignard reagents, and two molecules of 1,3-butadiene that affords a 1,6-octadiene carbon framework bearing alkyl and aryl groups at the 3- and 8-positions, respectively, and the competing cross-coupling reaction. Both the four-component coupling reaction and the cross-coupling reaction are triggered by the formation of anionic nickel complexes, which are generated by the oxidative dimerization of two molecules of 1,3-butadiene on Ni(0) and the subsequent complexation with the aryl Grignard reagents. The C–C bond formation of the alkyl fluorides with the γ-carbon of the anionic nickel complexes leads to the four-component coupling product, whereas the cross-coupling product is yielded via nucleophilic attack of the Ni center toward the alkyl fluorides. These steps are found to be the rate-determining and selectivity-determining steps of the whole catalytic cycle, in which the C–F bond of the alkyl fluorides is activated by the Mg cation rather than a Li or Zn cation. ortho-Substituents of the aryl Grignard reagents suppressed the cross-coupling reaction leading to the selective formation of the four-component products. Such steric effects of the ortho-substituents were clearly demonstrated by crystal structure characterizations of ate complexes and DFT calculations. The electronic effects of the para-substituent of the aryl Grignard reagents on both the selectivity and reaction rates are thoroughly discussed. The present mechanistic study offers new insight into anionic complexes, which are proposed as the key intermediates in catalytic transformations even though detailed mechanisms are not established in many cases, and demonstrates their synthetic utility as promising intermediates for C–C bond forming reactions, providing useful information for developing efficient and straightforward multicomponent reactions.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...