Rhodium-catalyzed asymmetric hydrogenation of β-cyanocinnamic esters with the assistance of a single hydrogen bond in a precise position†
Abstract
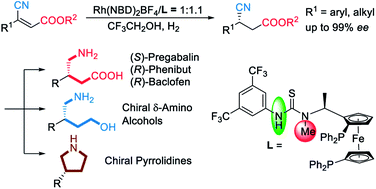
With the assistance of hydrogen bonds, the first asymmetric hydrogenation of β-cyanocinnamic esters is developed, affording chiral β-cyano esters with excellent enantioselectivities (up to 99% ee). This novel methodology provides an efficient and concise synthetic route to chiral GABA-derivatives such as (S)-Pregabalin, (R)-Phenibut, (R)-Baclofen. Interestingly, in this system, the catalyst with a single H-bond donor performs better than that with double H-bond donors, which is a novel discovery in the metalorganocatalysis area.


 Please wait while we load your content...
Please wait while we load your content...