Extraction of nickel from NiFe-LDH into Ni2P@NiFe hydroxide as a bifunctional electrocatalyst for efficient overall water splitting†
Abstract
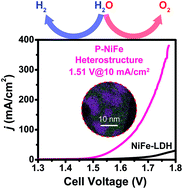
The development of highly efficient, low-cost and stable electrocatalysts for overall water splitting is highly desirable for the storage of intermittent solar energy and wind energy sources. Herein, we show for the first time that nickel can be extracted from NiFe-layered double hydroxide (NiFe-LDH) to generate an Ni2P@FePOx heterostructure. The Ni2P@FePOx heterostructure was converted to an Ni2P@NiFe hydroxide heterostructure (P-NiFe) during water splitting, which displays high electrocatalytic performance for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in 1.0 M KOH solution, with an overpotential of 75 mV at 10 mA cm−2 for HER, and overpotentials of 205, 230 and 430 mV at 10, 100 and 1000 mA cm−2 for OER, respectively. Moreover, it could afford a stable current density of 10 mA cm−2 for overall water splitting at 1.51 V in 1.0 M KOH with long-term durability (100 h). This cell voltage is among the best reported values for bifunctional electrocatalysts. The results of theoretical calculations demonstrate that P-NiFe displays optimized adsorption energies for both HER and OER intermediates at the nickel active sites, thus dramatically enhancing its electrocatalytic activity.
- This article is part of the themed collections: Most popular 2018-2019 materials chemistry articles, Most popular 2018-2019 energy articles, In celebration of Chinese New Year, Editors’ Choice Collection and 2018 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...