Total chemical synthesis of ester-linked ubiquitinated proteins unravels their behavior with deubiquitinases†
Abstract
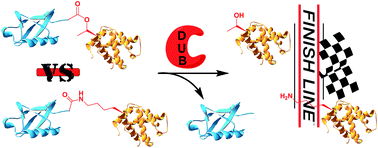
Ester-linked ubiquitinated proteins have been reported by several groups to be involved in ubiquitin signalling. However, due to the lack of the suitable tools to homogeneously produce such conjugates, their exact physiological roles and biochemical behavior remain enigmatic. Here, we report for the first time on the development of a novel synthetic strategy based on total chemical synthesis of proteins to construct ubiquitinated proteins, where ubiquitin is linked to the substrate via an ester bond. In this study, we prepared ester- and isopeptide-linked ubiquitinated α-globin and examined their relative behaviors with various deubiquitinases. We found that deubiquitinases are able to cleave the ester linkage with different efficiency relative to the isopeptide-linked substrate. These results may indicate that ester-linked ubiquitinated proteins are natural substrates for deubiquitinases.
- This article is part of the themed collection: Collection to celebrate our diverse and global authorship


 Please wait while we load your content...
Please wait while we load your content...